In June 2018, Cellular Microbiology, the internationally authoritative journal of bacteriology research, published online a breakthrough in the field of pathogenesis of Streptococcus suis serotype 2 (SS2) made by the research team led by Professor Fan Hongjie, of College of Veterinary Medicine, Nanjing Agricultural University. The title of the paper is the serine/threonine protein kinase of Streptococcus suis serotype 2 affects the ability of the pathogen to penetrate the blood brain barrier.
Streptococcus suis serotype 2 (SS2) is a zoonotic agent. Not only does it seriously damage the development of the pig industry, causing serious public health problems in Jiangsu and Sichuan Provinces in 1998 and 2005, respectively, with heavy casualties. SS2 can cause meningitis in humans and pigs, but the mechanism is unknown. Under the support of the National “973 Program” and the National Key R&D Program, two Ph.D. students, Liu Rui and Li Weiyi, of the team, used transposon (TnYLB-1) mutagenesis to discover that STKs/STPs may affect the expression of E3 ubiquitin ligase HECTD1 and subsequently increase the degradation of claudin-5, and thus enhance the permeability of the BBB. This study demonstrates for the first time that STKs/STPs of SS2 can degrade the tight junction protein claudin-5 of bEnd.3, and it reveals the mechanism of this degradation. The research results can further elucidate the pathogenic mechanism of SS2 and provide a theory for the discovery of new drug targets and vaccine development.
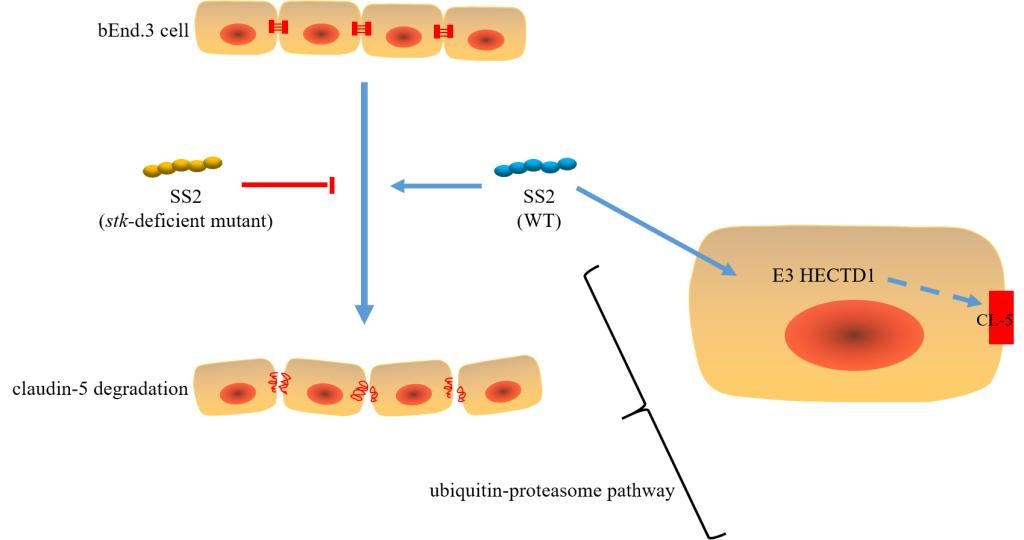
Schematic diagram of the mechanism of the degradation of bEnd.3 tight junction protein claudin-5 by STKs/STPs of SS2
The abstract of this paper is as follows:
Streptococcus suis serotype 2 (SS2) is a zoonotic agent that causes meningitis in humans and pigs. However, the mechanism whereby SS2 crosses the microvasculature endothelium of the brain is not understood. In this study, transposon (TnYLB-1) mutagenesis was used to identify virulence factors potentially associated with invasive ability in pathogenic SS2. A poorly invasive mutant was identified, and was found to contain a TnYLB-1 insertion in the serine/threonine kinase (stk) gene. Transwell chambers containing hBMECs were used to model the blood-brain barrier (BBB). We observed that the SS2 wild-type ZY05719 strain crossed the BBB model more readily than the mutant strain. Hence, we speculated that STK is associated with the ability of crossing blood-brain barrier in SS2. In vitro, compared with ZY05719, the ability of the stk-deficient strain (Δstk) to adhere to and invade both hBMECs and bEnd.3 cells, as well as to cross the BBB, was significantly attenuated. Immunocytochemistry using antibodies against claudin-5 in bEnd.3 cells showed that infection by ZY05719 disrupted BBB tight junction proteins to a greater extent than in infection by Δstk. The studies revealed that SS2 initially binds at or near intercellular junctions and crosses the BBB via paracellular traversal. Claudin-5 mRNA levels were indistinguishable in ZY05719- and Δstk-infected cells. This result indicated that the decrease of claudin-5 was maybe induced by protein degradation. Cells infected by ZY05719 exhibited higher ubiquitination levels than cells infected by Δstk. This result indicated that ubiquitination was involved in the degradation of claudin-5. Differential proteomic analysis showed that E3 ubiquitin protein ligase HECTD1 decreased by 1.5-fold in Δstk-infected bEnd.3 cells relative to ZY05719-infected cells. Together, the results suggested that STK may affect the expression of E3 ubiquitin ligase HECTD1 and subsequently increase the degradation of claudin-5, thus enabling SS2 to traverse the BBB.